8.4.1. Prescribing Controlled Drugs
When prescribing CDs, the prescriber must consider:
·
How the patient will benefit from being prescribed a CD
·
Potential risks with prescribing, for example, potential for overdose or
diversion
·
Medications the patient is currently taking, including non prescribed
medications
·
Opioid naive
·
Also refer to NHS Tayside Formulary as well as external sources such as
British National Formulary (BNF) and the National Institute for Health and
Care Excellence (NICE) for advice and guidance.
To meet legal requirements, the following details must be present (in
indelible ink) on the prescription. These requirements do not apply where
CDs are prescribed on the TPAR for administration to inpatients. If any of
the details are missing, the script is invalid and the Pharmacist
must be contacted.
·
The full name and address, including the post code of the patient. If the
patient does not have a fixed address, then No Fixed Abode is acceptable.
A PO Box or an email address is unacceptable.
·
The patient’s CHI number should be included
·
If a patient is aged 12 years or younger, their age and weight must be
included on the prescription
·
The dose to be taken must be written following Home Office guidelines and
state, one to be taken as directed / when required. Simply writing as
directed or when required is unacceptable.
·
The formulation must be stated. Using ‘caps’ or ‘tabs’ is acceptable,
however, please write in whole words for clarity
·
The strength must be written, even if only one strength is available. To
avoid ambiguity, where a prescription requires multiple strengths of a
medicine, each strength should be prescribed separately.
·
The total quantity must be written in both words and figures and should be
expressed as a number of dosage units, for example 2 tablets of 20mg
rather than 40mg total quantity. Liquids should be expressed as
millilitres (mL).
·
The quantity prescribed must not exceed 30 days. This is not a legal
restriction, however, the prescriber should be able to justify their
decision to prescribe more than 30 days’ supply.
·
If the CD prescription is written by a dentist, then the words for dental
treatment only must be present
·
Where the prescription is intended to be supplied in instalments, a valid
instalment direction is required
·
When the CD is supplied, it is a requirement to mark the prescription with
the date of supply at the time the supply is made. The prescription needs
to be written in indelible ink and can be computer generated.
Prescribing of Schedule 2, 3 and 4 CDs should be restricted to a maximum
of 30 day supply.
Community GP10 Prescription Form:
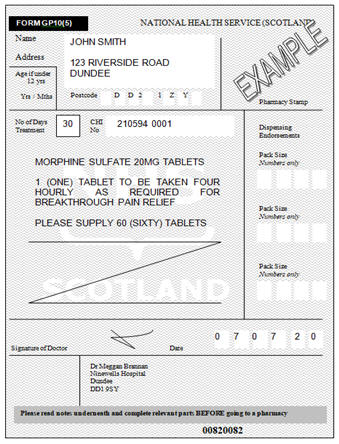

Regulations do allow for computer generated CD prescriptions, however, it
is essential they are checked and signed by the prescriber after printing
to minimise the risk of error.
Practitioners should not prescribe CDs for themselves, their colleagues, family or friends except in an
emergency.
8.4.2. Checking the
Dose
When opioid
medicines are prescribed in anything other than acute emergencies, the
healthcare practitioner concerned should:
·
Confirm any recent opioid dose, formulation, frequency of administration
and any other analgesic medicines prescribed for the patient. This may be
done for example through discussion with the patient or their
representative (although not in the case of treatment for addiction), the
prescriber or through medication records.
·
Ensure where a dose increase is intended, that the calculated dose is safe
for the patient
·
Ensure they are familiar with the following characteristics of that
medicine and formulation: usual starting dose, frequency of
administration, standard dosing increments, symptoms of overdose, common
side effects.
8.4.3. Portal
Discharge
The Portal Discharge Form can be accessed via Clinical Portal.
Where a supply of CD is required on an EDD
Prescription, a paper copy should be printed off and signed by the
Prescriber. This paper copy should be sent to Pharmacy for dispensing.
Pharmacists have the ability to edit entries
for CDs. Usual practice should remain that the Prescriber completes the
information required for CDs; Pharmacists can amend information in line
with advice in the BNF as long as this is relayed back to the Prescriber.
“In
the case of a prescription for a Controlled Drug in Schedule 2 or 3, a
Pharmacist can amend the Prescription if it specifies the total quantity
only in words or in figures or if it contains minor typographical errors,
provided that such amendments are indelible and clearly attributable to
the Pharmacist (e.g. name, date, signature and GPhC registration number)”.
8.4.4. Monitoring
Patients prescribed
controlled drugs should be monitored in accordance with relevant local and
national guidance
8.4.5. Validity
8.4.6. Technical
Errors on CD Prescription
Where a prescription for a Schedule 2 or 3 CD contains a minor
typographical error or spelling mistake, or where either the words or
figures (but not both) of the total quantity has been omitted, the
Pharmacist may amend the prescription indelibly so that it becomes
compliant with legislation.
The Pharmacist must be satisfied beyond reasonable doubt that:
·
They are satisfied that the prescription is genuine
·
They are satisfied that the drug is being supplied in accordance with the
intention of the prescriber
·
The prescription is amended so as to be indelible to correct the minor
typographical or spelling mistake, so the prescription complies with the
stated requirements
The amendments must be attributable to the Pharmacist, for example name,
date, signature and GPhC registration number.
Pharmacists cannot correct other amendments or omissions, for example,
missing date, incorrect dose, form or strength. These should be corrected
by the original prescriber, or in an emergency another prescriber
authorised to prescribe CDs. Amendments cannot be made by covering letter
from the prescriber.
8.4.7. Prescribing
for Patients to be Discharged or Transferred
Prescriptions for CDs for patients who are going home (discharge medicines) must be written on approved prescription forms for dispensing by the Hospital Pharmacy. These prescriptions must conform to all requirements of the Misuse of Drugs Regulations 2001 for a CD prescription.
For patient transfer, it is the responsibility of the discharging Ward to
inform the receiving Ward before transfer to allow appropriate
arrangements to be made for supply before the next dose is due. Ward stock
of CDs must not be transferred with the patient. This may require CDs to be
dispensed for a named patient, as part of a discharge prescription. (CDs
would only be supplied in this way if patient was transferred between
sites and the receiving unit did not have stock and couldn’t obtain a
supply in time from their own Pharmacy. This process would not happen if
the transfer was between Wards within the same site).This ensures the continuation of patient care in the receiving
Hospital, Ward or Department until that area can establish a Ward supply
from Pharmacy.
8.4.8. Prescribing
for Outpatients
CD prescriptions for outpatients must be written in accordance
with the requirements of the
Misuse of Drugs Regulations 2001
Regulation 15. The
prescription must be written on the approved outpatient prescription form
(for example,
headed notepaper) for the Hospital Pharmacy to dispense or a Hospital
Prescription for a Community Pharmacy to dispense and should conform to
all requirements.
Patients discharged in the Out Of Hours period when the
Hospital Pharmacy is closed, cannot not be given CDs from Ward stock.
If Doctor can write a HBP10 Form for CDs,
advise the patient to visit Community Pharmacy to obtain supply.
8.4.9. Prescribing in Instalments
An instalment direction combines two pieces of information:
1. Amount of medicine per instalment
2. Interval between each time the medicine can be supplied
The first instalment must be dispensed within
28 days of the appropriate date. The remainder of the instalments should
be dispensed in accordance with the instructions (even if this runs beyond
28 days after the appropriate date).
If the only date on the prescription is the
date of signing, the first dispensing needs to take place within 28 days
of this date. If the prescriber indicates on the prescription a date
before which the prescribed medicine should not be dispensed, this would
be the appropriate date instead. The prescription must then be marked with
the date of each supply.
The instalment direction is a legal
requirement and needs to be complied with. Because there are acknowledged
practical difficulties with missed doses and dates when the Pharmacy is
closed, for example, Bank Holidays, the Home Office has approved specific
wording to be used that gives Pharmacists a degree of flexibility when
making a supply.
Approved wording:
1. Please dispense instalments
due on Pharmacy closed days on a prior suitable day
2. If an instalments collection
day has been missed, please still dispense the amount due for remaining
day(s) of that instalment
3. Consult the prescriber if
three or more consecutive days of a prescription have been missed
4. Supervise consumption on
collection days
5. Dispense daily doses in
separate containers