8.8.1. Destruction of Controlled Drugs
All Ward stock CDs under safe custody which are no longer required (have
reached their expiry date or patientís own) must not be returned to
Pharmacy but should be destroyed at Ward / Department level by two
registered healthcare professionals, where one must be a Pharmacist or
Pharmacy Technician.
Please refer to local SOPs for the destruction of CDs which are not under
safe custody restrictions. Please note, that these CDs can be destroyed by
two registered healthcare professionals, where one must be a Pharmacist or
Pharmacy Technician at Ward / Department level.
The Controlled Drug Register (where applicable) should be completed accordingly with the date, time, reason for destruction and the quantity destroyed. Each entry should be signed by the two members of staff involved. Ensure that the register balance matches physical balance. The CD team must be notified of any discrepancy and a DATIX entry submitted.
Please note that Schedule 2 Controlled Drugs are all subject to
restrictions on possession and supply and require secure storage
(alongside Schedule 3 Burpenorphine and Temazepam).
It is the responsibility of the Ward / Department to ensure they
have appropriately sized / quantity of CD Destruction Kits for
destruction.
All Controlled Drugs must be rendered irretrievable prior to disposal using a denaturing kit (see Section 8.8.4.).
For destruction of Controlled Drugs (all schedules), see
table for guidance (see
Section 8.8.2.
8.8.2. CD Destruction within NHS Tayside Hospital Wards and Community
Hospitals ONLY
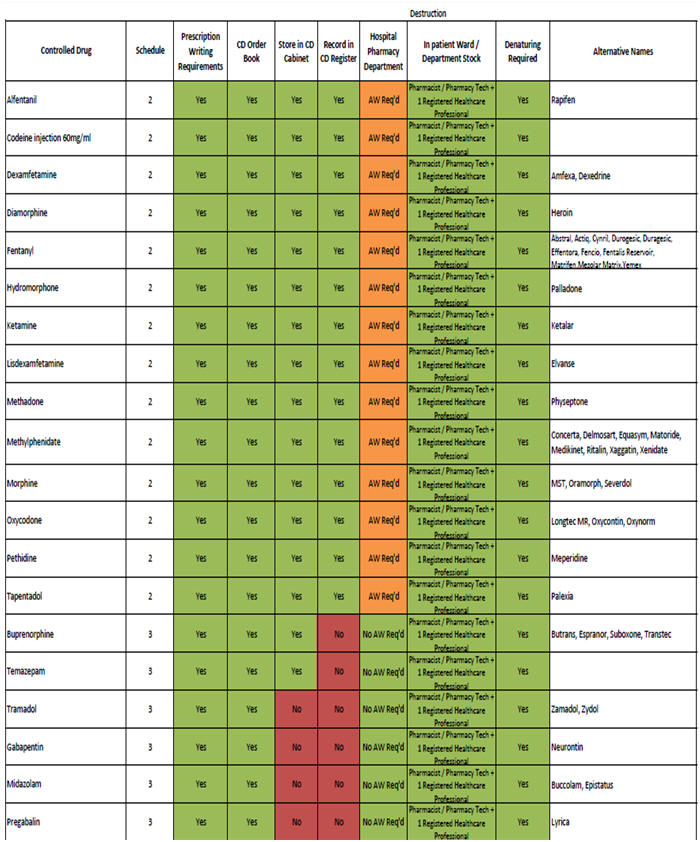
8.8.3. CD Destruction within NHS Tayside GP Practices and Community Pharmacies
In Hospital Pharmacy, Community Pharmacy and General Practice CDs can only
be destroyed in the presence of an Authorised Witness (person who has
signed authorisation from Controlled Drugs Accountable Officer). For the
destruction of Schedule 2 Controlled Drugs (within Community Pharmacy and
General Practitioner settings), please contact
tay.cdteam@nhs.scot
to arrange a destruction. It is the
responsibility of the Community Pharmacy / GP Practice to ensure they have
appropriately sized / quantity of CD Destruction Kits for destruction.
8.8.4. Controlled
Drugs Types and their Destruction Process
∑
Aerosols:
Remove the metal collar from around the top of the bottle using
appropriate equipment. Remove
the bottle cap which
includes the entire pump assembly. Use a pipette or syringe to remove the
contents into a denaturing kit. Double rinse the container and pour into
the destruction kit. Following double rinsing the bottles can have their
labels removed and placed into appropriate waste streams for uplift. Or
blue lidded pharmaceutical waste container.
∑
Ampoules:
Remove ampoule from packaging, ensure no liquid in the top of the vial by
flicking the top and ensure it
falls to the base of the
ampoule. Break the ampoule at the weak point and aspirate its contents
using a syringe or pipette before dispensing into the destruction kit.
Place the empty ampoule into the destruction kit. If this is not possible,
the empty ampoule must go into a blue lidded pharmaceutical waste
container. The syringe or pipette must be disposed of appropriately,
either into the destruction kit (size dependant) or into the blue lidded
pharmaceutical waste container.
∑
Capsules:
Pull apart both ends of the capsule and empty its contents into the
destruction kit. Place both
ends of the capsule shell in the destruction kit.
∑
Concerta:
Remove the tablet from its packaging and fully submerge the tablets in
boiling water (in a suitable container) until it has fully melted. Add all
of the liquid and the remaining part of the tablet to the denaturing kit.
∑
Liquids:
Measure the volume in a
measuring cylinder (if bottle is opened, if the bottle is sealed, use
manufacturer volume). Remove the lid from the bottle and pour its contents
into the destruction kit. Double rinse the container and measuring
cylinder and pour into the destruction kit. Following double rinsing the
bottles can have their name and signage deleted and placed into
appropriate waste streams for uplift. Or Pharmaceutical waste bin.
∑
Lozenges:
Remove the lozenge from its packaging and fully submerge in boiling water
(in a suitable receptacle) and melt. Add all of the liquid to the
denaturing kit.
∑
Patches:
Remove the patch from its packaging and peel off the protective coating.
Fold the patch onto itself several times ensuring no drug is exposed.
Place the folded patch into the destruction kit.
∑
Powdered injections:
Reconstitute with water to dissolve powder. Aspirate its contents using a
syringe or pipette before dispensing into the destruction kit. Place the
empty vial into the destruction kit. If this is not possible, the empty
vial must go into a Pharmaceutical waste bin.
∑
Sachets:
Hold the sachet and flick the packet to ensure the powder falls to the
base of the sachet. Carefully open over the destruction kit and then empty
its contents into the destruction kit, ensuring that all contents have
been emptied. Place the empty packaging into the destruction kit. If this
is not possible, the packaging must go into a Pharmaceutical waste bin.
∑
Suppositories:
Remove the suppository from its packaging and place into boiling water (in
a suitable receptacle) and melt. Add all of the liquid to the denaturing
kit.
∑
Tablets:
All must be crushed. Remove tablet (s) from packaging and place in a
tablet crusher or Mortar. Use the Pestle to crush the tablet if using a
Mortar. All tablets must be completely crushed into a fine powder in which
no larger pieces can be retrieved. Pour the crushed contents into a
destruction kit. Remove as much of the powder which may be stubborn and
remain on the bottom of the Mortar using a spatula. Rinse the Mortar with
water and dispose that water into the destruction kit.
∑
After all CDís have been appropriately destroyed and placed into the
denaturing kit, water should then be added. Place the lid on securely and
shake vigorously (over a sink), therefore, activating the solution and
rendering the drugs irretrievable. Once the contents have congealed, the
destruction kit should be placed into a blue lidded pharmaceutical waste
container and then sealed to avoid any diversion of the kit.
All Controlled Drugs must be rendered irretrievable prior to disposal.
This will require the use of a Pestle and Mortar, syringe/pipette or
boiling water to destroy each before being placed into a destruction kit.
Please ensure appropriate PPE is worn during the destruction. The sequence
in which items should be destroyed is:
∑
Tablets
∑
Capsules
∑
Patches
∑
Sachets
∑
Ampoules
∑
Aerosol
∑
Lozenges
∑
Suppositories
∑
Liquids
8.8.5. Destruction
of Patientís Own CDs in a Hospital Ward or Department
Where patients have brought their own CDs into Hospital with them and they
are no longer required, or if a labelled ĎPatientís Owní supply has been
made by Pharmacy and is no longer required, these CDs can be destroyed by
2 registered healthcare professionals, where one must be a Pharmacist or
Pharmacy Technician at Ward level (with patientís consent):
CDs which have not left the Dispensary but have been labelled for patient and remain uncollected remains as Pharmacy stock, therefore, must be destroyed in the presence of an Authorised Witness. Patientís Own CDs on the Ward do not need to be destroyed in the presence of an Authorised Witness (see Section 8.8.2.).
∑
CDs must be rendered irretrievable
before disposal using a CD Destruction kit
∑
A record of the quantities destroyed must be made in the appropriate page
of the CD Register or ĎPatientís Owní CD Record Book of the CDs destroyed
and must be signed by both parties
∑
The used CD Destruction kit must be placed in a blue-topped medicines
waste bin.
∑
It is safer for
patients prescribed CDs, who are dealing with many
medicines, to go home with only medicines they currently require to manage
their symptoms.
The Patientís Own Controlled Drug Register should be retained for a period
of seven years.
There is no legal requirement for patient returned Controlled Drugs
requiring safe custody to
be destroyed in the presence of an Authorised Witness.
8.8.6. Destruction
of Remainders of Continuous Infusions Containing CDs
When a continuous infusion containing a CD is commenced, the total amount
in the prepared infusion is considered as ďadministeredĒ as far as the
entry in the CD Register is concerned.
If the infusion is discontinued before all of
the solution has been infused, the amount remaining must be checked by two
registered healthcare practitioners where one must be
a Pharmacist or Pharmacy Technician, or a registered healthcare
professional and a suitably trained competent witness if available against the TPAR, and must then be discarded into a pharmaceutical waste
bin containing a Vernagel sachet.
It is recognised that there may only be one registered professional
available and therefore, local policies must be referred to.
The destruction must be witnessed, and both staff involved
must sign on the designated part of the infusion record chart.
Complete the CD Register and annotate clearly.
8.8.7. Disposal of
Unused Part of CD Doses
This applies only to CD doses that are to be discarded after
being prepared for administration to a patient.
Discarded part of CD doses should be rendered irretrievable on disposal (the contents of the ampoule must be expelled on top of the Vernagel in the blue lidded pharmaceutical waste container. Placing a full ampoule into the blue lidded pharmaceutical waste container containing CDs is not acceptable) (see Section 8.8.4.).
Liquids up to a maximum volume of 5mL,
including part-used ampoules, syringes etc, should be dispensed into a
blue lidded pharmaceutical waste container following the addition of
Vernagel. Vernagel is an absorbent polymer which solidifies liquids. The
Vernagel sachet should be placed into the bottom of the blue lidded
pharmaceutical waste and any medicines to be disposed of should then be
added.
Larger volumes require the use of a CD Destruction Kit. One
kit can be used for several discards during a session.
8.8.8.
Breakages/Spillages/Dropped Doses
All breakages, spillages and dropped tablets must be reported to the registered healthcare professional in charge of the Ward / Department as soon as possible and reported via DATIX.
Breakages, spillages and dropped tablets must be entered in the Ward /
Department CD Register (example not inclusive of Theatre CD
Register)running balance, explaining the reason for the discrepancy
between actual stock and the amount shown in the CD Register with the
signature of a witness, for example:
25-APR-2020 1 x 5mg ampoule
Diamorphine broken by Nurse A (signature); witnessed by Nurse B
(signature)
NAME, FORM OF PREPARATION AND
STRENGTH....DIAMORPHINE
5mg AMPOULES
7
|
AMOUNT(S) OBTAINED |
AMOUNT(S) ADMINISTERED |
||||||||
|
Amount
|
Date Received |
Serial No
Of
Requisition |
Date |
Time |
Patientís Name |
Amount given |
Given by
(signature) |
Witnessed by
(signature) |
STOCK BALANCE |
|
Carried
forward from Page Number.......6......
Balance on
transfer |
|||||||||
|
|
|
|
24/04/2020 |
18.30 |
BALANCE B/F FROM PREVIOUS PAGE |
|
Nurse A |
Nurse B |
20 |
|
|
|
|
25/04/2020 |
0230 |
Stock Check |
|
Nurse A |
Nurse B |
20 |
|
|
|
|
25/04/2020 |
1035 |
Dropped Vial. Segregated for destruction |
1 x 5mg |
Nurse A |
Nurse B |
19
(+1 for CDD) |
|
|
|
|
25/04/2020 |
1038 |
Patient A |
1 x 5mg |
Nurse A |
Nurse B |
18
(+1 for CDD) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8.8.9. Refused Doses
Any refused doses can be destroyed on the Ward / Department (example not
inclusive of Theatre CD Register) by 2 registered members of staff. The CD
Register must be updated accordingly.
NAME, FORM OF PREPARATION AND STRENGTH....METHYLPHENIDATE 20mg TABLETS
5
|
AMOUNT(S) OBTAINED |
AMOUNT(S) ADMINISTERED |
||||||||
|
Amount
|
Date Received |
Serial No
Of
Requisition |
Date |
Time |
Patientís Name |
Amount given |
Given by
(signature) |
Witnessed by
(signature) |
STOCK BALANCE |
|
Carried
forward from Page Number.......4......
Balance on
transfer |
|||||||||
|
|
|
|
24/04/2020 |
18.30 |
BALANCE B/F FROM PREVIOUS PAGE |
|
Nurse A |
Nurse B |
72 |
|
|
|
|
25/04/2020 |
0235 |
Stock Check |
|
Nurse A |
Nurse B |
72 |
|
|
|
|
25/04/2020 |
0923 |
Patient A Refused Dose Destroyed |
1 x 20mg |
Nurse A |
Nurse B |
71 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carried over to page number ..........
8.8.10. Out of Date (OOD)/Expired CDs
Out of date / expired CDs must be segregated in the CD Cabinet. A new
entry in the CD Register must now show the quantity available and the
number OOD / expired, for example, 20 + (5 OOD
OR EXP). The (5 OOD
OR EXP) must be completed in
every line until the OOD stock has been destroyed. Although the stock has
been segregated, no assumptions must be made during stock checks, a
physical stock check must take place of all OOD CDs even if stored in a
sealed bag or container. (Ward / Department CD Register, not Theatre CD
Register)
NAME, FORM OF PREPARATION AND STRENGTH....MORPHINE SULPHATE MST 60mg TABLETS
15
|
AMOUNT(S) OBTAINED |
AMOUNT(S) ADMINISTERED |
||||||||
|
Amount
|
Date Received |
Serial No
Of
Requisition |
Date |
Time |
Patientís Name |
Amount given |
Given by
(signature) |
Witnessed by
(signature) |
STOCK BALANCE |
|
Carried
forward from Page Number.......14......
Balance on
transfer |
|||||||||
|
|
|
|
24/04/2020 |
18.30 |
BALANCE B/F FROM PREVIOUS PAGE |
|
Nurse A |
Nurse B |
20 (+5 OOD) |
|
25/04/2020 |
0235 |
Stock Check |
|
Nurse A |
Nurse B |
20 |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carried over to page number ..........