Adverse drug reaction reporting
Report all serious suspected reactions to established drugs and report all suspected reactions (including those considered not to be serious) to drugs showing the black triangle symbol (▼) (usually newly licensed medicines).
An adverse drug reaction (ADR) is an unwanted or harmful reaction experienced following the administration of a drug or combination of drugs under normal conditions of use, which is suspected to be related to the drug. The reaction may be a known side effect of the drug or it may be new and previously unrecognised. Rapid detection and recording of adverse drug reactions is vital so that hazards are identified promptly and appropriate regulatory action is taken to ensure that medicines are used safely. Suspected ADRs to any therapeutic agent should be reported, including drugs (self-medication as well as those prescribed), blood products, vaccines, radiographic contrast media, complementary and herbal products.
Healthcare professionals are urged to report suspected ADRs directly to the Medicines and Healthcare products Regulatory Agency (MHRA) through the Yellow Card Scheme using the electronic form at http://yellowcard.mhra.gov.uk/. Alternatively Yellow Card reporting forms can be found after the index within the BNF or can be downloaded from the MHRA website: http://www.mhra.gov.uk/. UK Medicines Information pharmacists can submit completed adverse drug reaction enquiries from their in-house database directly to the MHRA yellow care scheme.
The Yellow Card Scheme relies upon voluntary reporting of suspected adverse drug reactions. Further details on alternative methods of reporting and further advice and information on suspected ADRs can also be found on the ‘Adverse drug reactions’ page in the Safety information section of the MHRA website.
Patients and their carers can also report suspected ADRs to the MHRA. Reports can be submitted directly to the MHRA through the Yellow Card Scheme using the electronic form (http://yellowcard.mhra.gov.uk/) or by obtaining a patient Yellow Card from a pharmacy or GP surgery. Further information for patients and details of other methods of self-reporting ADRs is available on the ‘Patient reporting’ page of the MHRA website.
Established drugs
Healthcare professionals are asked to report all serious suspected reactions to established drugs (including over-the-counter, herbal, and unlicensed medicines and medicines used off-label) and vaccines. Serious reactions include those that are:
They should be reported even if the effect is well recognised. A severe reaction might not be life-threatening or disabling but can seriously affect an individual patient. For example, headaches are not normally considered serious in nature, but may be very severe. A severe or exaggerated ADR should be reported.
Newer drugs
A Black Triangle (▼) is assigned to a product if the drug is an active substance which has been newly licensed for use in the UK. However, a product containing previously licensed active substances may also be monitored and assigned Black Triangle status if it meets one or more of the following criteria:
All similar biological medicines (biosimilars) have a Black Triangle symbol because every new biological product has been developed to be similar to an existing biological product, however may not have an identical structure therefore requires intensive monitoring for safety and efficacy. Healthcare professionals are asked to report all suspected adverse reactions (including those considered not to be serious) associated with Black Triangle products, in order to:
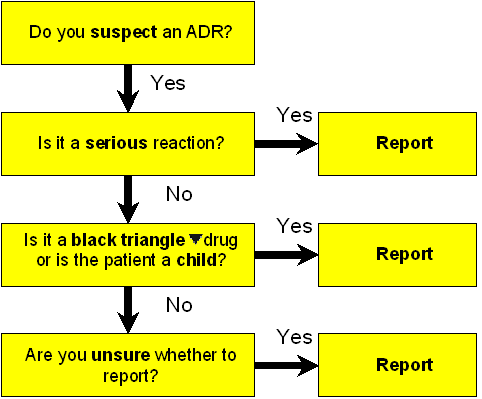
The following reporting reminder flowchart may be useful in helping to decide whether to report an ADR (if in doubt – report):

© 2010 NHS Tayside