8.3.1. Controlled Drug Stationery - Definition
Controlled Stationery is any stationery, which, in the wrong hands could
be used to obtain medicines fraudulently.
Once in the Ward / Department / Theatre in the Hospital or other
healthcare premises, the person receiving (for example, Senior Charge
Nurse / Midwife/ Operating Department Practitioner) the Controlled
Stationery is responsible for its security.
A record shall be kept in Pharmacy or by Primary Care Services of the receipt and issue of Prescription Forms and Controlled Drug Order Books, including the date issued and the identity of the person requesting and issuing it. Only one Controlled Drug Order Book must be held on a Ward / Department at any one time except when otherwise agreed locally to meet exceptional circumstances, for example, Community Hospitals. The completed book will be returned to the Ward from the Pharmacy Department for retention for 2 years following the last completed order of CDs supplied. Records shall be periodically examined by Pharmacy staff for inconsistencies and any anomalies investigated. (see Section 8.7.2.)
Pharmacy issue new HBP10 Forms and CD Order Books when Ward / Department
have less than 5 pages remaining in the respective stationery. The Ward /
Department must prove the numbers of pages remaining before these are
issued.
Loss or theft of any Controlled Stationery shall be reported immediately to Senior Charge Nurse / Midwife / Operating Department Practitioner or Ward Pharmacist and reported on DATIX.
Unused Controlled Drug Order Books and Prescription Forms must be returned
to the issuing Pharmacy Department or Primary Care Services, where their
receipt must be recorded.
Records of the receipt and issue of Controlled Stationery will be retained
securely for 2 years in the relevant clinical area.
CD Order Book must be stored in the CD Cabinet. CD Order Books pertaining
to the Ward / Department must be delivered to Pharmacy by a member of
their Ward / Department team.
CD Register and Patient’s Own CD Register must be stored where practical
in the CD Cabinet. Where this is not possible, it must be kept in a secure
area that is locked, for example, a locked Cabinet or drawer where access
is restricted. For ‘High
flow areas’, such as A&E, refer to Local Policies regarding Controlled
Stationery.
All Controlled Stationery must be available for inspection at all times.
Controlled Drug Registers that are
being replaced (see Section 8.3.5.)must have part used pages ruled off using a ‘lazy Z’
and the CD Register
should be archived securely for 2 years and then destroyed by shredding or
confidential waste. Any CD Register containing patient’s own destruction
is to be kept for 7 years and destroyed as previous. The date of the last
entry should be written on the front cover to make it easier to establish
when the CD Register can be destroyed.
Controlled Drugs Registers and Order books shall be held for 2 years (7 years for a CD Register containing Patient Own CDs which have been destroyed) from the date of last entry, in accordance with the Misuse of Drugs Regulations 2001. (See Section 8.3.12.)
The Senior Nurse / Midwife is responsible for the requisitioning of CDs
for use in that area and for ensuring that all Controlled Stationery used
to order, return or distribute CDs is stored securely and that access to
the stationary is restricted to those staff authorised to order CDs.
8.3.2. Request and Supply of Controlled Stationery
Wards and Departments must only have one CD Order Book in the Department
at any time, with the exception of Community Hospitals where an additional
emergency CD Requisition Book may be required.
A
request form for a CD
Register or CD Order Book can be obtained from Pharmacy.
A registered Nurse/Midwife must complete the request form
New CD Registers/Order Books cannot be issued without a written request
Only one CD Order Book must be given at any given time
If a CD Order Book or CD Register is misplaced or lost, a DATIX must be entered.
8.3.3. Missing Controlled Stationery
Loss or theft of any Controlled Stationery must be reported immediately to
Senior Charge
Nurse / Midwife / Operating Department Practitioner in charge of the Ward
/ Department / Theatre. The Senior Charge Nurse / Midwife must:
·
Ensure items are genuinely missing. Check CD Cabinet, recent
administration records etc (this may need to be done discreetly)
·
Inform Senior Charge
Nurse / Midwife or Lead Pharmacist
at earliest opportunity. Must be within 24 hours. Out of hours senior
manager on call and the Pharmacist on call should be notified and an
immediate investigation started.
·
Obtain replacement stationery to allow continued treatment
· Escalation to the CD Team by submitting a DATIX incident report form.
8.3.4. Controlled Drug Registers
Each Ward / Department that holds stocks of CDs must keep a record of CDs
received and administered in the CD Register or Patient’s Own CD Register.
The Senior Charge Nurse / Midwife / Operating Department
Practitioner is responsible for keeping these CD Registers up to date and
in good order. CD Register and Patient’s Own CD Register must be stored
where practical in the CD Cabinet. Where this is not possible, it must be
kept in a secure area that is locked, for example, a locked Cabinet or
drawer where access is restricted. For ‘High
flow areas’, such as A&E, should refer to Local Policies regarding
Controlled Stationary.
All entries must be signed by a registered nurse or midwife and best
practice would be that this is countersigned witnessed by a second
registered nurse, midwife. If any of the secondary staff mentioned are
unavailable, then the transaction can be witnessed by another registered
practitioner or a suitably trained competent witness if available.
Only one CD Register must be in use for recording Ward stock at any time,
unless under exceptional circumstances, for example, large amounts of CDs
in use.
Where an area accepts Patient’s Own CD, a Patient’s Own CD Register must
be used for this purpose.
Entries in all CD Registers must be made in black ink and must be
indelible (markings that
cannot be removed). Red ink should only be used for stock level
checks by Pharmacy staff or error corrections.
Entries must be made in chronological order with no empty lines between
entries (Wards and Departments only, not Theatre CD Register)
The drug, form and strength must be written clearly and legibly at the
head of each page. Each
different drug must be written on a
separate page, for example Morphine Sulfate 10mg (Modified Release)
tablets on a separate page from Morphine Sulfate 10mg Immediate Release
tablets. Entries for both receipt and supply
for each drug
preparation should be made on the
same page, enabling best practice in the maintenance of running balances.
On reaching the end of a page in the CD Register, the balance must be
transferred to another page. The new page number must be recorded in the
index in the front of the CD Register corresponding to the appropriate
drug, form and strength. The new page number must be annotated at the
bottom of the finished page. “Carried forward from” relevant page number
must be inserted at the top of the new page.
Entries must be clear and unambiguous. Entries and errors must never be
altered or obliterated. Correction fluids should also never be used. Any
errors must be bracketed and the correct entry made in an adjacent space
or next line. A brief explanation, for example, entered in error must be
made in the margin or at the bottom of the page and then signed and dated.
Pages or part pages must never be torn out of the CD Register.
Writing in the CD Register must be the last task to be completed when, for
example, when CDs are dispensed, they must not be signed out of the CD
Register until they leave the Dispensary and are in the possession of the
Ward / Department / Theatre or Patient.
All CD Registers must be kept
securely (see Section 8.3.12.) for two years (7 years for a CD Register containing Patient
Own CDs which have been destroyed) from the date the last entry was made,
and then disposed of as confidential waste.
8.3.5. Closing or Starting a Controlled Drug Register
Closing a CD Register:
When closing a CD Register, use a ‘Lazy Z’ to close off all incomplete
pages. On the external front cover of the CD Register write the date of
the last entry made in the CD Register and the retention time this should
be kept for.
Write the words “CD Register closed DD-MMM-YYYY (date). Balances
Transferred to CD Register Serial Number (which can be found on the inside
of the new CD Register)”.
Starting a New CD Register:
Write the CD, strength and formulation on the Index page of the CD
Register with the corresponding page number.
Enter the date the CD Register is opened. Balances transferred from CD
Register, including serial number (serial numbers not present in Patient’s
Own CD Registers).
On the top of the page, write the name of the CD, strength and
formulation.
Complete the date, time and write ‘Balance Carried Forward’, update
‘Running Balance’ and then sign this entry.
Repeat for all CDs recorded within this CD Register.
8.3.6. Controlled Drug CD Register Discrepancy Recording
NAME, FORM OF PREPARATION AND STRENGTH....OXYCODONE
20mg TABLETS
5
|
AMOUNT(S) OBTAINED |
AMOUNT(S) ADMINISTERED |
||||||||
|
Amount
|
Date Received |
Serial No
Of
Requisition |
Date |
Time |
Patient’s Name |
Amount given |
Given by
(signature) |
Witnessed by
(signature) |
STOCK BALANCE |
|
Carried forward from Page Number.......4......
Balance on
transfer |
|||||||||
|
|
|
|
24/04/2020 |
18.30 |
BALANCE B/F FROM PREVIOUS PAGE |
|
Nurse A |
Nurse B |
72 |
|
|
|
|
25/04/2020 |
0235 |
Stock Check |
|
Nurse A |
Nurse B |
72 |
|
|
|
|
25/04/2020 |
0922 |
Patient A |
1 x 20mg |
Nurse A |
Nurse B |
71 |
|
[ |
|
|
25/04/2020 |
0925 |
Patient B |
1 x 20mg |
Nurse A |
Nurse B |
70
]
|
|
|
|
|
25/04/2020 |
0930 |
LINE ABOVE WRITTEN IN ERROR – PATIENT B SHOULD NOT HAVE BEEN
DISPENSED 1x 20mg |
1 x 20mg SEGREGATED FOR DESTRUCTION |
Nurse A |
Nurse B |
70
(+1 for CDD) |
|
|
|
|
|
|
|
|
|
|
|
When an error is made
in the CD Register, the erroneous entry must not be cancelled, obliterated
or altered in any way, however, an entry must be made stating the error.
(Wards and Departments CD Registers only, not Theatres CD Register)
An entry can be made
if the error is noted at the time, as above, or by way of a marginal note
or footnote which must specify the date on which the correction is made.
An * can be used to highlight the line in which there is an error
and the clear explanation must be annotated if there is not a line
available immediately underneath the line of error.
Please note: CDD refers to Controlled Drug Destruction. (See Section 8.8.1)
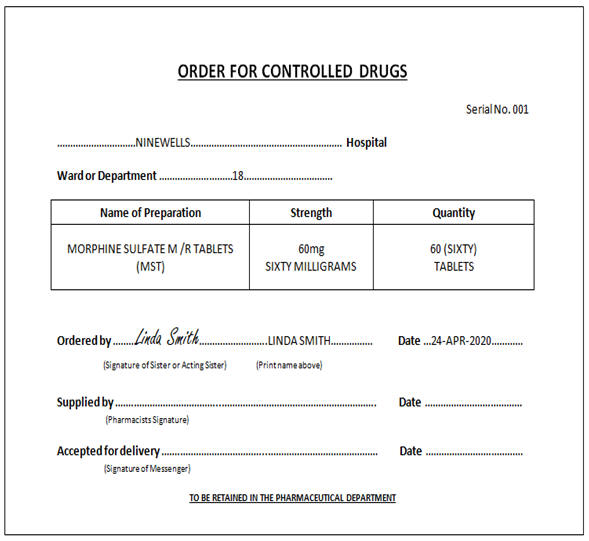
8.3.7. Controlled Drug Order Book
CDs must be ordered in the CD Order Book, ensuring that a carbon paper is
inserted between the top white and pink copy sheet. Block capitals must be
used stating the name of the CD, strength, form and quantity required
(words and figures). A
separate page must be used for each preparation ordered.
The order must have the signature and printed name of the registered
healthcare professional authorised to order CDs; initials are not
acceptable.
If necessary, a registered Pharmacist or Pharmacy Technician may have to
alter quantities supplied, for example, part pack or blister strip or a
complete pack or to clarify formulation when supplied. Where this happens,
the quantity stated must be altered, with the quantity change in both
words and figures, signed and dated on both copies of the CD requisition
by the Pharmacy member making the alteration. Pharmacy staff must contact
the Ward / Department who has placed the order to discuss any changes to
their order before it is assembled in Pharmacy. The member of staff
consulted must be documented on the order page. Where other aspects are
incorrect, the Ward must be contacted to correct the order. Corrections
can be made by the Ward Pharmacist or Senior Charge Nurse / Midwife and
signed accordingly.
Where a patient requires a CD that is not stocked by Pharmacy, Pharmacy
must contact the Ward / Department to discuss an alternative supply.
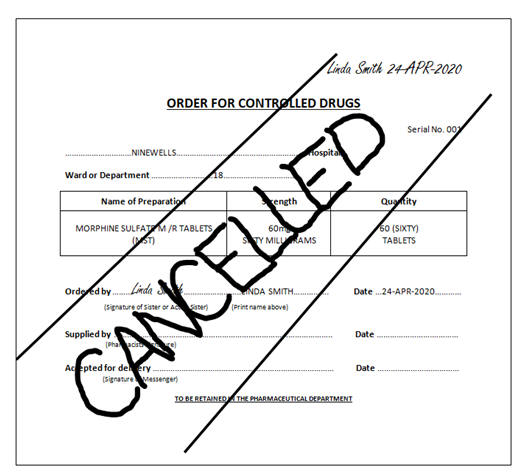
Cancelled orders must not be torn out of the CD Order Book.
The cancellation must appear on both the white original and the pink copy
of the CD Order Book.
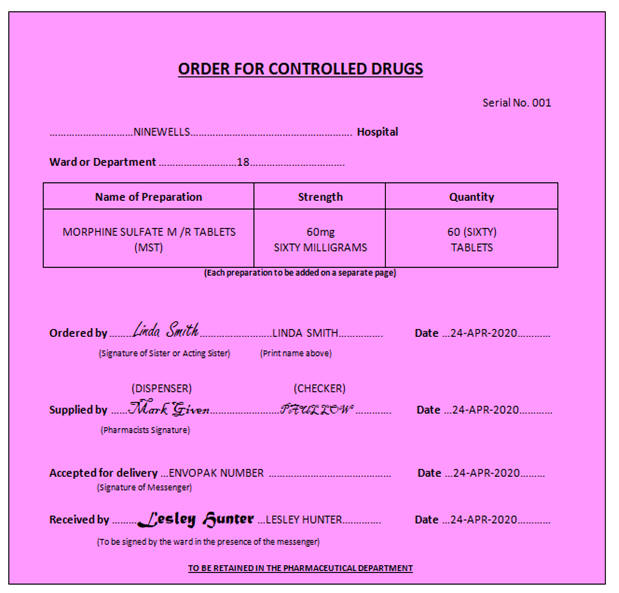
8.3.8. Receiving
Controlled Drugs - Controlled Drug Order Book
When CDs are delivered onto the Ward / Department, a registered Nurse must
check the contents of the order immediately and write through the CD
Register, witnessed by a second Registered Nurse, Pharmacist or Doctor. As
a matter of good practice and whenever possible, the person ordering the
CDs should be different to the person receiving the CDs into stock.
The CDs should then be locked away in the CD Cabinet.
Receipt of orders must be confirmed by the Nurse signing the relevant pink page in the CD Order Book.
8.3.9. Ordering New Controlled Drug Order Book
When a Ward / Department require a new CD Order Book, a Registered
Healthcare Professional from that Ward / Department will approach Pharmacy
hatch and complete
CD Order Book
Request form.
The Registered Healthcare Professional from Ward / Department requiring
new CD Stationery will take the completed Book to show Pharmacy the CD
Order Book is complete. There should be no more than one CD Order Book in
circulation for the Ward / Department (unless there is a specific reason –
approval will be sought from the Ward / Department Pharmacist). The
complete CD Order Book is returned to the Registered Healthcare
Professional and stored on the Ward / Department.
Once the Registered Healthcare Professional completes the form, a member
of Pharmacy staff will sign out the CD Stationery requested and the
Registered Healthcare Professional will sign for this. At this point, a
Stock Check of Controlled Stationery is undertaken by a member of the
Pharmacy Team.
CD Order Book Request Forms to be archived (see Section 8.3.12.) in the Pharmacy Department for a minimum of 2 years.
8.3.10.
Controlled Stationery Request Form
8.3.11. Documentation of Receipts in the
Controlled Drug Register
·
Amount written in digits and words. Words to be written in capitals.
·
Record the date in which the CDs are received
·
Record the serial number of requisition. This is
also the page number in the CD
Order Book.
· Record the, the serial tab number of the envopak. Both persons must check this and ensure it has not been tampered with. This number should collate with the CD Order Book. If these numbers do not match, an investigation should begin to ascertain why. A DATIX must be submitted
·
Record the time and where the CDs have been
received from.
·
Both members of staff must check the order against the delivery and the
running stock balance.
·
Signatures represents that staff have completed all the relevant checks.
· Any discrepancy or concern must be reported immediately to the supplying Pharmacy and recorded on DATIX.
·
CDs must never be unattended.
·
CDs that require safe storage must be immediately placed into the CD
Cabinet on delivery following appropriate signage of paperwork with
Pharmacy staff. Details of CDs
(agreed by local policies and procedures to be recorded in a CD Register)
must be entered in the CD Register as soon as practically possible.
|
NAME, FORM OF PREPARATION AND STRENGTH....MORPHINE SULFATE MST 10mg TABLETS
9 |
|||||||||||
|
AMOUNT(S) OBTAINED |
AMOUNT(S) ADMINISTERED |
||||||||||
|
Amount
|
Date Received |
Serial No
Of
Requisition |
Date |
Time |
Patient’s Name |
Amount given |
Given by
(signature) |
Witnessed by
(signature) |
STOCK BALANCE |
||
|
Carried forward from Page Number.......8......
Balance on
transfer |
|||||||||||
|
|
|
|
24/04/2020 |
1830 |
BALANCE B/F FROM
PREVIOUS PAGE |
|
A Nurse |
B Nurse |
20 |
||
|
60 (SIXTY) |
24/04/2020 |
001 |
Envopak No.79003125 |
1831 |
RECEIVED FROM PHARMACY |
|
A Nurse |
B Nurse |
80 |
||
|
|
|
|
|
|
|
|
|
|
|
||
8.3.12.
Retention Times for CD Records
|
Document |
Retention Time |
Derivation of
Recommendation |
|
Requisition Sheets |
2 Years |
Misuse of Drugs Regulations 2001 |
|
External Orders and Delivery Notes |
2 Years |
Misuse of Drugs Regulations 2001 |
|
CD Order Book |
2 Years |
Misuse of Drugs Regulations 2001 |
|
CD Ward Orders or Requisitions |
2 Years |
Misuse of Drugs Regulations 2001 |
|
Copy of signature for Ward CD Ward Order or
Requisition |
Duration of Employment |
|
|
CD Registers |
2 Years from Last Entry |
Misuse of Drugs Regulations 2001 |
|
CD Registers containing Destructions |
2 Years from Last Entry |
Misuse of Drugs Regulations 2001 |
|
Patient’s Own CD Registers containing
Destructions |
7 Years from Last Entry |
|
|
CD Stock Check |
2 Years from Last Entry |
|
|
Extemporaneous Preparation Worksheet |
13 Years |
GMP, but consider keeping for longer due to
consumer liability legislation |
|
Aseptic Worksheet (Adult) |
13 Years |
NHS Code of Practice Part 2 Second Edit |
|
Aseptic Worksheet (Paediatric) |
26 Years |
NHS Code of Practice Part 2 Second Edit |
|
Prescriptions |
2 years |
Misuse of Drugs Regulations 2001 |
|
Clinical Trials |
25 Years |
Good Clinical Practice |
|
Invoices |
6 Years |
Controlled Drugs: Safe Use and Management |
|
CD Transport via road vehicle |
Driver ID – minimum 3 months Recipient signature, documents
– minimum 6 months All requisitions, signed
orders, orders and private prescriptions – 2 years |
Guidance for the Safe Custody of Controlled
Drugs and Drug Precursors in Transit |