8.5.1. Controlled Drug Stock
The Ward/Department should have a list of the CDs to be held in each
Ward/Department as stock items, including minimum stock levels. The
contents of the list must reflect current patterns of usage of CDs in the
Ward/Department and must be agreed between the Pharmacist or Pharmacy
Technician responsible for stock control of medicines and the Senior
Charge Nurse/Midwife.
The list must be modified if practices change and must be subject to
annual review.
Where possible, routine orders should be placed on Monday - Friday and not
on weekends/public holidays.
8.5.2. Daily
Controlled Drugs Stock Check
The Senior Charge Nurse / Midwife / Operating Department Practitioner is
responsible for ensuring
that daily stock checks are performed once every 24 hours. The stock
balance of CDs, (tablets, capsules, injections and patches) must be
physically counted by two registered members of staff. If this cannot be
done, complete a DATIX report. Patientís Own CDs must be checked and
signed for during the daily stock check
The CD check must be carried out by two registered healthcare
professionals or one registered nurse and one student nurse / midwife.
8.5.3. Procedure for Daily CD Stock Checking
Checking of CD stocks must include:
∑
Checking the running balance in the CD Register against the contents of
the CD Cabinet, not the reverse, to ensure that all balances are checked.
∑
Ward staff carrying out their daily check should physically check the
balance of all CDs, for example, tablets, capsules, injections and patches
against that documented in the CD Register to ensure they are the same but
do not write anything in the Register.
∑
The best time for performing the Daily CD Stock Check is at handover
between dayshift and nightshift.
∑
The two members of staff then sign the daily check sheet to confirm that
they have carried out the check once finished checking the contents of the
cupboard
∑
It is not necessary to open packs with tamper evident seals but the
integrity of all packaging and products must be assessed.
∑
Oral liquid preparations of CDs should not be physically measured at every
CD check. Instead, a visual estimation approach is an acceptable method.
If a discrepancy is suspected a registered healthcare professional may
undertake a physical measurement of open
bottles of liquid CDs.
Routine periodic volume measurement checks should be implemented by
Senior Charge Nurse / Midwife / Operating Department Practitioner
to ensure volumes recorded in the CD Register are accurate and reflect
actual stock balance. (It should be
assumed that manufacturer sealed bottles contain the amount stated on the
label). Plastic cups are not appropriate for accurately measuring CDs.
(Contact pharmacy if advice is required on measuring CD
liquids accurately)
∑
The balance for liquids must be confirmed on completion of a bottle. The
balance should be adjusted / agreed once a bottle has been finished.
Bottles which contain a shortened expiry date once opened must be
physically measured if the expiry date has passed before completion of the
bottle.
∑
A record of any reconciliation must be made in the CD Register.
∑
Expiry dates must be checked.
∑
If patientís own CDs are stored then these must also be checked.
∑
Ensure that there is adequate stock and re-order if required.
A record of all CD stock checks must be made in the appropriate
documentation.
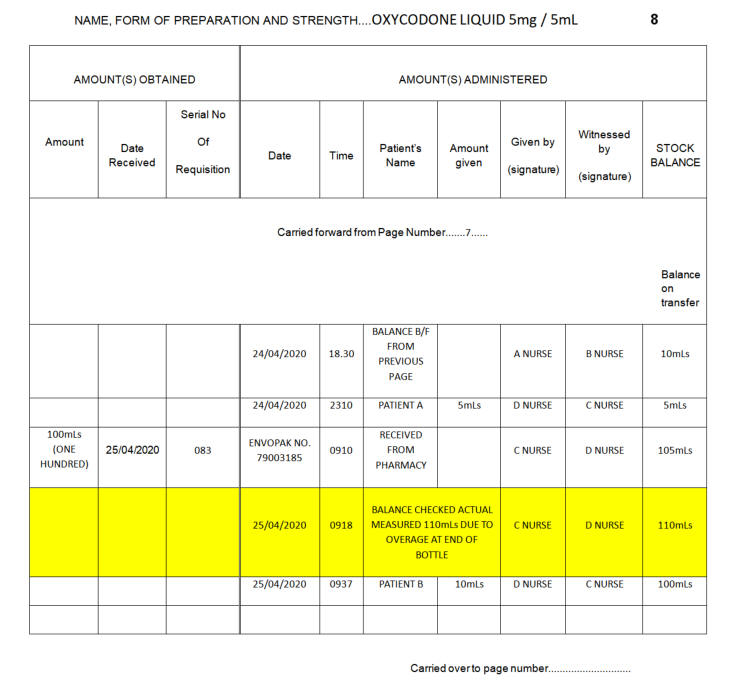
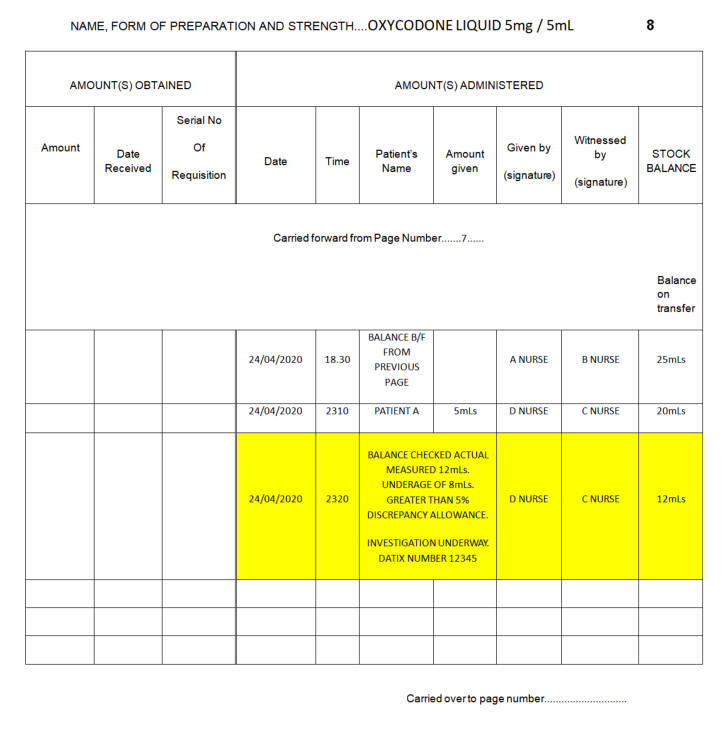
If an error or omission can be explained the registered healthcare professional must make an entry in the CD Register clearly stating the reason for the entry and the correct balance. This entry must also be witnessed and signed by a second registered member of staff. An unexplained discrepancy must be reported immediately to the Senior Charge Nurse or Midwife in charge. A DATIX must then be logged following this verbal communication.
Never leave an unlocked CD Cabinet unattended at any time.
8.5.4. Four Monthly CD Audit by Pharmacy Staff
A check of all CDs stocked within Wards / Departments must be completed
every four months by a member of Pharmacy (Pharmacist / Pharmacy
Technician) and a registered Nurse.
The Pharmacist / Pharmacy Technician performing the stock check with the
registered member of staff (Nurse)
must check the volume of all liquid CDs. These must be checked visually
and only measured using an appropriate calibrated measure. Plastic
medicine cups are not suitable for accurately measuring CDs.
When measuring liquid CDs, ensure:
∑
Place the measuring cylinder on a flat, hard surface
∑
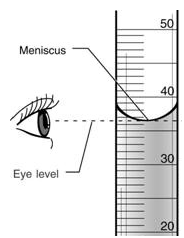
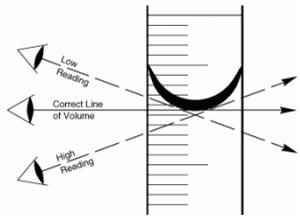
Eye line is at the same height as the bottom of the meniscus
∑ Bottom of the meniscus is the accurate measurement
If the stock level has not been rebalanced by staff every time they open
a new bottle, even if the volume appears visually accurate, you must
measure all opened bottles using an appropriate calibrated measure and
annotate the actual stock balance in the appropriate CD Register.
Measuring Liquid CD Dose for Administration to Patient:
∑
Use an oral dose 5mL syringe to measure the
required dose
∑
Always use the syringe with the bottle adaptor described above
∑
Allow the patient to take the dose directly from the syringe or put the
measured dose in a medicine cup to administer to the patient
∑
Double rinse out the medicine cup and oral syringe with water
∑
Dispose of medicine cup and syringe in general waste
8.5.6. Minimise
Loss of Liquid CDs
To reduce / minimise loss of liquid CDs when measuring doses for
administration to patients, it is advised that an adaptor is used for each
bottle of liquid CDs as follows:
∑
Insert Baxter Healthcare Bottle Adaptor
into the top of the bottle of liquid CD
∑
Keep this bung in situ until the bottle is empty or the contents have
expired
∑
Replace the cap on bottle each time it is used
∑
Double rinse the bung and cap then dispose of in general waste stream once
no longer required
A liquid discrepancy is defined as +/-5% of
the volume contained in the bottle.
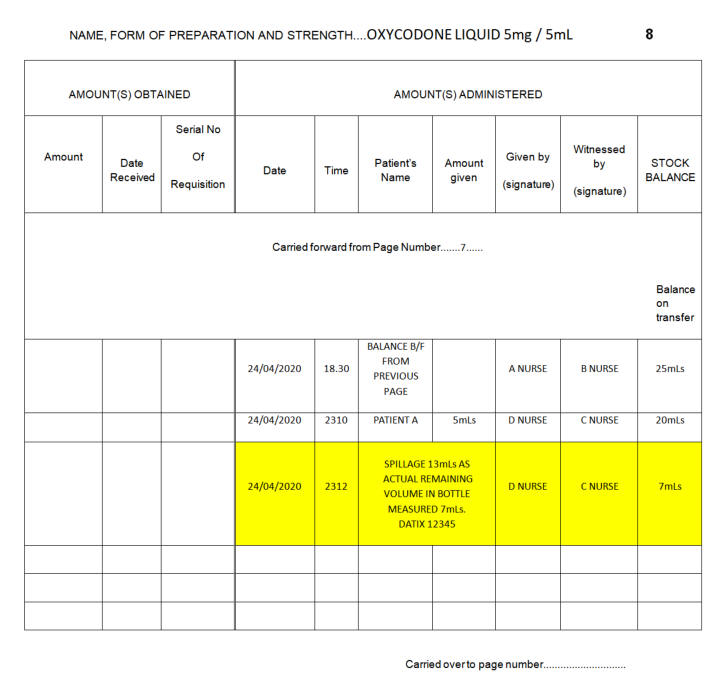
8.5.7. Liquid CD
Spillages
When spillages occur, a witness is required to
verify that the spillage has occurred and this should be recorded in the
CD Register (as process for
breakages/spillages/dropped doses section
-
see Section 8.8.8.)
and signed by both the person making the spillage and the witness. To
accurately record the volume lost, the remaining volume in the bottle
should be measured and the total balance should reflect what volume
remains. A
DATIX must be submitted
following any loss or breakage of CDs.
For large liquid CD spillages, for example,
Methadone, use paper towels to soak up the liquid from the surface. The
used paper towels should then be placed into a blue lidded pharmaceutical
waste container and Vernagel placed over the used paper towels. The used
paper towels must not be disposed of in normal waste streams or rinsed
down the sink.
In place of residence, for example, patients own home, nursing home, care
home, group home, this person may be a Health Care Support Worker,
relative, carer or the patient.
8.5.8. Controlled
Drug Discrepancies
The balances in the CD Register must always tally with the amounts of CDs
in stock. If they do not, the
discrepancy must be fully investigated
as soon as possible (see
Section 8.7.2.). In the
first instance the following should be carefully checked:
∑
Arithmetic since last correct balance.
∑
Re-check CD Cabinet or GP bag with second person (remember to include date
expired stock and exclude patient returns which may have become mixed with
stock).
∑
Other CD Register sections of same drug class for erroneous entries.
∑
Other holdings, for example, GP bags for stock which may have been
transferred but not recorded.
∑
Sense-check register (correct pack sizes, patterns of entry for potential
missing entries, and unusual
quantities).
∑
All orders have been entered into the CD
Register by checking CD Register and CD Order Book on the Ward /
Department as well as delivery notes / invoices / stock orders within
Pharmacy for discrepancies.
∑
All CDs administered have been entered into
the CD Register. This would involve checking every patientís TPAR.
∑
Check diary and contact all relevant
practitioners who have worked at the Ward / Department during the relevant
period to verify any supplies made that may have not been entered or been
entered erroneously.
If the error or omission is traced, Senior Charge Nurse /
Midwife / Operating Department Practitioner must make an entry in the CD
Register clearly stating the reason for the entry and the corrected
balance. This entry must also be witnessed by another healthcare
professional (not student nurse) and both must sign the CD Register.
A liquid discrepancy is defined as + / -5% of the volume contained in the bottle.
8.5.9. How to
Calculate Discrepancies
How to calculate 5%:
Balance (mL) x 5 = 5%
100
Example calculation:
Register = 60mL
100
*Remember discrepancy can be a shortage or an overage
If the discrepancy cannot be resolved, the Senior Charge Nurse or
Midwife in charge must make an entry in the CD Register clearly
documenting the discrepancy (including the actual balance) and stating
that this is under investigation. The entry must be witnessed by
another healthcare professional and both must sign the CD Register.
8.5.10. Clinical
Trials
Where a Controlled Drug is being used in a clinical trial, all CD requirements (including, prescription, supply and storage) remain applicable and supersede any trial arrangements. The clinical trial must comply with the Misuse of Drugs regulations and local policies governing the management of clinical trial medicines, in addition to clinical trials legislation and MHRA guidance.
All clinical trial CDs should be stored separately from stock
CDs, however, do not need to be stored in a separate CD Cabinet.