8.15.1. Patientís
Own Controlled Drugs (CDs)
Unless returned to the patientís
relative(s)/representative(s) immediately, Patientís Own Schedule 2 and 3
CDs requiring safe storage (Section
8.2.3) must be identified on
admission and removed with their consent for safe storage.
Patientís Own CDs may be
used providing they have been assessed (via a POD assessment) by the Nurse
before administration Ė also refer to:
Medicines Management 1 (use of Patientís Own
Medicines).
Patientís Own CDs must be
written in the Patientís Own CD Register and stored in the Ward /
Department CD Cabinet immediately and be administered in accordance with
the NHS Tayside local policy, Safe and Secure Handling of Medicines.
Where Patient's Own CDs
requiring safe storage (including Hospital Pharmacy discharge or Ďpassí
medications labelled for a patient) are stored or used, a Patientsí Own CD
Register must be used for this purpose. The record must include the
following:
-
A record of the patientís name, CHI and date of entry.
-
Name, form, strength and quantity received of the CD. The volume must be physically measured and recorded for opened bottles of liquid CD.
-
The signature of two registered members of staff one of whom is a registered nurse whilst the other may be a Doctor, Pharmacist, Nurse/Midwife or student Nurse/Midwife, Pharmacy Technician or Operating Department Practitioner
-
Where appropriate any remaining spaces must be short ruled (so no further entries may be added to the page) following return, completion or destruction of the CDs. Following closure of the page within the Patientís Own CD Register, close the corresponding page number on the index page
8.15.2. Transfer of Patient's Own CDs
If a Patient is transferred between
Ward/Department, the Patientís Own CDs must be signed out of the original
Patientís Own CD Register and entered into the receiving Ward / Department
Patientís Own CD Register. Ward/Department Controlled Drug Transfer Form
must be used (Section 8.9.5).
8.15.3.
Stock Check of Patient's Own CDs
Patientís Own CDs should be checked during the
daily stock check (once every 24 hours) by checking the Index and balance
for each patient in the Patients Own CD Register against the contents of
the CD Cabinet, not the reverse, to ensure that all balances are checked.
Refer to (Section 8.5.3).
Any discrepancies must be investigated immediately (Section
8.7)
If there is a query or concern regarding CDs in a
monitored dosage system stored in the CD Cabinet, the Ward / Department
Pharmacist / Pharmacy Technician should be contacted for clarification.
8.15.4.
Patient Discharge
At the time of discharge, if there are no changes
to the patientís CD prescription, for example, no changes to dose,
strength or form, the Patientís Own CDs should be returned to the Patient
and an entry to that effect shall be made in the Patientís Own CD
Register. It is
safer for
patients to go home
with only prescribed medicines.
When a patient leaves the clinical area, the
record in the Patientís Own CD Register must be completed indicating
whether the CDs were transferred to another clinical area with the
Patient, returned to the Patient or relative / representative or
destroyed. Regular checks should be carried to ensure this has been
completed. The closure of the page on the index as per process above will
also act as a separate check.
8.15.5 Examples of how to complete Patient's Own CD Register
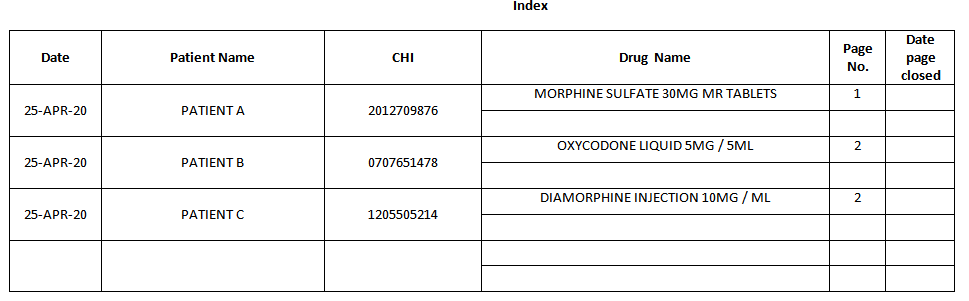
Index:
All Patientís Own CDs
must be recorded in the Index section of the Patientís Own CD Register
when they arrive on the Ward / Department. The first five columns must be
completed and the details of each CD recorded on the relevant page in the
CD Register.
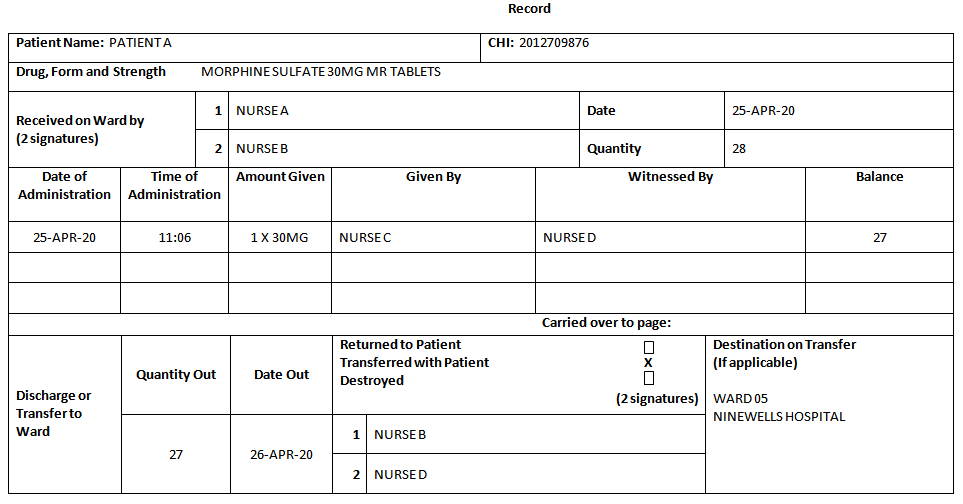
Record:
Add the details of the Patientís name and CHI; the name, form, strength
and quantity of the CDs received. Receipt should be acknowledged by 2
registered members of staff. At each dose administration, complete the
relevant columns.
At the end of the Patientís stay, indicate whether the remaining amount
has been Returned to Patient,
Transferred with Patient or
Destroyed and complete the
remaining columns of the section.
Section 8.8.5 must be followed for the disposal and destruction of any
Patientís Own CDs.
8.15.6. Controlled
Drug Storage in a Patientís Home
A local risk assessment must be carried out before agreeing that CDs can
be left in a patientís home.
Patients and their family/carer should be given instruction on how to
store prescribed CDs securely within their own home. They should not be on
display to others and therefore unlikely to be misused or diverted. Advice
should be provided by the practitioner issuing the prescription and the
Community Pharmacist who is supplying the CDs to the patient/family/carer.
Nurses must also advise patients and their family/carer regarding the safe
storage of their medication in accordance with the patient information
leaflet and instructions on the label.
If the decision is made that it is unsafe to leave large amounts of CDs in
the patients home, other measures must be put in place, for example, daily
collection from Community Pharmacy.
If a practitioner becomes aware that
CDs are missing
from a patientís home (see
Section 8.7.1.), this must be reported immediately and a
DATIX
report submitted and an
investigation
started.